- About
- Solutions
- Governance
- Investors
- Sustainability
- Sustainability
- Newsroom
- Jobs
As recycling has a significant impact on decarbonising the refractory industry, RHI Magnesita is pursuing various initiatives to increase reusing refractory products. This includes establishing MIRECO, the key partner for a circular supply chain. In general, reclaimed refractory material comprises two categories: Pieces that are >80 mm and a fine fraction, which accounts for ~40%. Although in the last years efficient practices have been developed to process the larger material and incorporate it into new refractory products, currently it is uneconomical to sort the fines. Therefore, approaches to sustainably utilise this material, which has historically been landfilled, are integral for the recycling strategy. This paper describes one of the most important ways to valorise this fine material in the steelmaking industry, namely as circular metallurgical additives. Since the carbon footprint of these additives can be <10% of commonly used slag formers, using this material contributes to a steel plant’s sustainability goals. To maximise the advantages of applying circular additives, RHI Magnesita can also provide metallurgical consulting and slag engineering tools. To illustrate these services, the article focuses on specific e-tech modelling tools available in the Customer Portal for basic oxygen furnace (BOF) slag optimisation, which are based on isothermal stability diagrams that combine MgO solubility at a specific basicity and temperature. A real case scenario is also provided to highlight the numerous benefits that can be achieved when circular metallurgical additives are applied in the BOF.
The extraction and processing of refractory raw materials are both energy intensive and can cause geogenic emissions, resulting in savings of approximately 2 tonnes of CO2 equivalent (CO2e) emissions for every tonne of refractory material recycled [1]. Therefore in 2022, to support decarbonisation initiatives, RHI Magnesita’s recycling activities were integrated with Horn & Co. Minerals Recovery, under the company name MIRECO [2]. While the current annual recycling stands at 160000 tonnes, this joint venture is pivotal for RHI Magnesita’s sustainability strategy of increasing recycling utilisation, with a target of achieving 270000 tonnes per year by 2026. After use, spent material is dismantled and various approaches are in place to maximise the amount that can be utilised in new refractory products. However, as a significant portion of the reclaimed material is finer than 80 mm (i.e., ~40%) and currently uneconomical to sort, it is being used for other applications, including circular metallurgical additives, rather than being landfilled with the associated costs and environmental impact.
This paper describes RHI Magnesita’s ongoing initiatives to support the application of circular additives through sourcing strategies, metallurgical consulting, and slag engineering tools, with a focus on the basic oxygen furnace (BOF).
Metallurgical additives are integral in iron and steelmaking as the slag’s chemical composition influences how it reacts with the liquid metal during refining. Slag components are divided into two groups: Basic oxides (e.g., CaO and MgO) and fluxing oxides (e.g., SiO2, Al2O3, MnO, and FeO). These compounds can be added to the slag intentionally, created through the oxidation of metallic elements in the scrap and hot metal (i.e., oxygen blowing), and/or derived from refractory lining wear. Slag engineering involves achieving the appropriate ratio of these two oxides groups required for a specific metallurgical process using additives. For example, dephosphorisation and desulphurisation reactions require CaO to be dissolved in the slag and while in the BOF CaO solubility is enhanced by FeO, in the ladle it is fluxed by SiO2, Al2O3, and CaF2. Additionally, a MgO saturated slag is necessary to prevent MgO-carbon refractory lining wear. This summary aims to illustrate the complexity of slag engineering, as well as highlight that the chemistry of metallurgical additives must be tailored to the specific process and optimised to control and/or reduce dissolution of the refractory lining.
Metallurgical additives are applied in numerous areas, from serving as MgO carriers for the sinter bed in blast furnaces to acting as fluxing agents in secondary metallurgy. They also function as slag formers in the BOF, electric arc furnace (EAF), and will be critical for future electric smelting furnace processes. Due to the diverse range of applications, more than 40 sustainable additives are currently available from MIRECO that can be customised to meet each customer’s specifications and more than 45 steel plants in Europe are already using these green slag agents. In particular, integrated steel mills have a high demand for metallurgical additives as well as typically producing a substantial amount of spent refractory material. Therefore, MIRECO not only operates six European recycling plants for sorting and processing spent refractory material, but because these green additives are sensitive to freight costs, onsite steel plant solutions have also been established in Germany, with more in the planning stages.
The use of MIRECO’s circular metallurgical additives contributes to environmentally friendly steel production as the product carbon footprint (PCF) of these materials can be <10% of commonly used slag formers (Table I) and is detailed in the technical data sheet. Additionally, these sustainable products are characterised by their cost competitiveness, as they do not require calcination and are not dependent on fuel price volatilities. In terms of chemistry, the MgO content protects a basic refractory lining from corrosion, while carbon is an energy source as well as a reducing and/or slag foaming agent. When compared to synthetic premelted slags, recycled alumina products have the advantage of containing accompanying oxides that lower the melting point, making them a competitive alternative for calcium aluminate slags or even a CaF2 substitute.
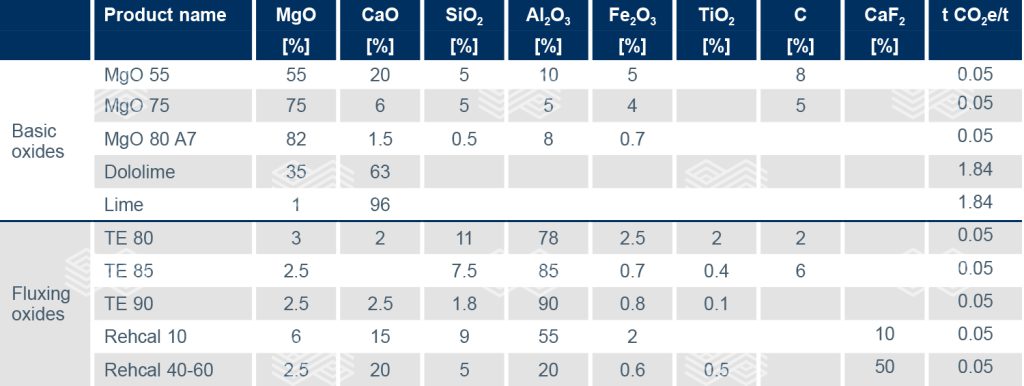
Table I. Examples of MIRECO’s circular metallurgical additives, which can be adjusted to each customer’s specifications, and standard slag formers. Abbreviations include tonnes of CO2 equivalent per tonne of metallurgical additive (t CO2e/t). Figures for dololime and lime are assumptions based on the best available technique reference documents.
As circular metallurgical additives are applied in significant quantities and with high frequency, the resulting carbon emission savings can be substantial. This is why an increasing number of steel plants are eager to adopt these sustainable additives. Nevertheless, there can be uncertainties associated with this approach including existing biases against using reclaimed materials, freight cost sensitivity, and the need for appropriate dissolution. However, laboratory and industry studies have demonstrated good solubility of circular additives in the EAF, even for MgO oversaturated slags [3].
In RHI Magnesita’s Customer Portal, refractory consumption and the associated scope 3 CO2e emissions can be tracked in real time for individual steelmaking units and on a monthly basis (Figure 1). This digital service can also be customised to visualise the CO2e savings that are achieved when circular metallurgical additives are being used, providing the customer with transparency regarding their decarbonisation potential. Another advantage of the Customer Portal is access to the slag modelling toolbox, known as e-tech [4,5]. e-tech streamlines and facilitates the use of circular additives by offering optimisation tools for CaO or MgO single saturation as well as dual saturation (Figure 2). In addition, there are tools for slag splashing and desulphurisation.
In summary, the diverse applications and sustainable impact on steelmaking processes makes offering circular metallurgical additives an extension beyond the typical refractory business, while complementing it. Not only is the chemistry tailored to a specific application, but customers can also be provided with a comprehensive consultancy service regarding their optimal application, including access to the e-tech slag modelling tools. This integration of circular metallurgical additives and slag engineering solutions propels RHI Magnesita further along the value chain and the aim is to serve as an interface between original equipment manufacturers (OEM) and steelmakers, to provide comprehensive process improvements and refractory
life cycle management.
In the following sections the metallurgical consulting service is exemplified by describing the modelling tools available for BOF slag engineering and the benefits that can be achieved when circular metallurgical additives are used in the converter.
During oxygen blowing, the addition of slag formers (e.g., lime, dololime, dolomite, limestone, and circular metallurgical additives) to the BOF is primarily determined by the hot metal silicon content and other metallurgical considerations such as dephosphorisation, manganese levels, thermal balance, and the required tap composition. In addition, an appropriate MgO slag saturation is necessary to minimise refractory lining wear. At later stages in the BOF process, maintenance methods such as slag rocking and splashing are widely adopted to protect the lining from subsequent scrap charging and hot metal impact, as well as chemical and mechanical wear in the trunnion areas. The required slag viscosity and adhesion characteristics for these practices depend on temperature and the precipitated solid phases. When determining the amounts of additives necessary to achieve an appropriate slag chemistry for these requirements, one approach is to prioritise MgO saturation.
Several proposed models are available to calculate MgO saturation as a function of complex slag composition and temperature based on saturation experiments conducted with various metallurgical process slags [6–12], of which a number provide MgO saturation as a function of slag basicity [7,9,11]. Other approaches involve deriving saturation diagrams from published phase diagrams in the CaO-MgO-FeO-SiO2-Al2O3 system [7] or utilising phase equilibrium software [13,14]. Table II provides an overview of published MgO slag saturation models for various metallurgical units.
Isothermal stability diagrams (ISDs) combine MgO solubility at a specific basicity (e.g., B3) and temperature. They are derived from the CaO-MgO-SiO2-FeO system [6,7] and have enjoyed widespread acceptance in the steelmaking community for several decades. Primarily used for optimising EAF and ladle slags, there are numerous case studies in the field that have substantiated the validity of these semi-empirical models. Furthermore, through FactSage simulations, involving complex slag compositions within the 6-component system CaO-MgO-FeO-SiO2-Al2O3-MnO specifically tailored to real BOF slag compositions, the use of ISDs has been extended to calculating MgO saturation in BOF slags.
RHI Magnesita’s e-tech software tool “Quick Foam” (also termed “Foamy Slag”), which is based on the previously described research [6,7], can be used to visualise the ISD as well as calculate the theoretical MgO saturation and other related figures for BOF slags.
The dependence of MgO solubility on slag basicity, temperature, and CaO-MgO-SiO2-FeO phase relationships is used to construct the ISD [6]. Figure 3 shows the ISD of a customer’s slag with a B3 of 2.6 and the stability regions of the various phases at 1660 °C. In addition to the liquid phase there are two stable solid mineralogical phases—MgO·FeO magnesiowüstite (MW) and dicalcium silicate (C2S).
The e-tech Quick Foam application enables insights into BOF slag chemistry using the ISD. The software visualises the analysed MgO value (blue square), the calculated MgO value (green triangle), and the theoretical MgO saturation point (red dot) based on input data such as slag chemistry, additions, and temperature (see Figure 3). The calculated MgO point results from the amount and chemistry of slag formers applied.
The difference between the analysed and calculated MgO values (i.e., Δ MgO) shows the amount of MgO pickup in the slag from the refractory brick lining and gunning mix, which must be avoided. The difference between the analysed MgO value and theoretical MgO saturation point (i.e., MgO supersaturation) indicates the slag’s affinity to dissolve MgO. The MgO supersaturation is positive or negative depending on whether the slag is over or undersaturated, respectively.
Based on the Quick Foam application, different trial scenarios for each customer’s requirements can be set up and adjusted to close the gap between the analysed and calculated MgO values. This generates a slag with less affinity to dissolve MgO, thereby minimising MgO pickup from the lining and gunning mix.
The most efficient approach for BOF maintenance involves adjusting the slag chemistry during the blowing process so the appropriate phases (i.e., MW and C2S) precipitate when the BOF temperature decreases after tapping. However, there are several considerations during blowing that can make MgO saturation inappropriate at this stage. In such cases, the residual slag after tapping can be adjusted for proper maintenance using the e-tech “Slag Splashing” tool (Figure 4), which calculates the flux additions necessary for ideal slag adhesion and refractoriness. Initially, the slag’s liquidus temperature at the end of blowing is determined from its chemical composition and temperature. Then the program outputs the weight of flux additions required to decrease the slag’s liquidus temperature by 75 °C, thereby generating an appropriate slag viscosity for BOF maintenance as the solid phases precipitate when the temperature decreases.
To practically investigate the topic of BOF slag engineering using circular additives and slag modelling tools, a series of trials were conducted at a steel plant. These investigations involved the use of circular additives in both sieved and briquetted forms at various stages of the process. The trials observed the dissolution of MgO in a highly dynamic BOF slag, along with the FeO reduction capacity of carbon-containing additives. A total of 140 slag samples were collected during these trials that underwent analysis using X-ray fluorescence, X-ray diffraction, optical light microscopy, and selected samples were also examined using scanning electron microscopy with energy dispersive spectrometry. As was previously demonstrated in the study evaluating the solubility of different circular MgO materials in EAF slags [3], it was also shown that both the sieved and briquetted materials dissolved effectively in BOF slags. Furthermore, although there was an increased MgO carrier addition, the total cost of ownership (TCO) calculation revealed a six-digit annual saving in euros, which included the following advantages:
Circular metallurgical additives deliver financial, environmental, and metallurgical benefits for steel producers, when appropriately processed and applied. Currently, MIRECO employs a large-scale circular raw material procurement and processing approach, resulting in three business cases that enable customers to take advantage of green additives (Figure 5). The first is direct sales, which targets providing customised additives for different process demands at a competitive price. With the “CERO Waste” opportunity, MIRECO offers recycling expertise such as how customers can optimally reuse their refractory fines for different metallurgical process demands and thereby reduce landfill costs as well as free up storage space. In the third option, the focus is on enhancing the steelmaking process with RHI Magnesita providing comprehensive metallurgical consulting for optimal circular additive application. For example, the slag modelling toolbox, e-tech, facilitates slag optimisation and maintenance adjustment with the Quick Foam and Slag Splashing applications performing calculations based on widely accepted and newly validated models for BOF slag engineering. Recently, a case study conducted at a BOF plant illustrated the practical applicability of circular additives in combination with slag modelling tools and resulted in financial benefits calculated from a TCO perspective.
[1] RHI Magnesita 2022 Sustainability Report. https://ir.rhimagnesita.com/wp-content/uploads/2023/03/42705-rhi-sustainability-report-2022-web.pdf
[3] Kek, F., Griessacher, T., Bauer, C., Zocratto, B., Krump, R. and Koubek, C. Refractory Waste to Slag Engineering Solution—Metallurgical Consulting Supports Steel Plant’s Circular Economy Strategy. Bulletin. 2022, 21–28.
[4] Souza, D., López, F., Moggee, H. and Lamare, C. A Toolbox of Slag Modelling and Metallurgy in Your Pocket. Bulletin. 2021, 72–77.
[5] https://etech.rhimagnesita.com/
[6] Pretorius, E.B. Introduction to Slag Fundamentals. Process Technology Group, LWB Refractories. https://etech.rhimagnesita.com/
[7] Pretorius, E.B. and Carlisle, R.G. Foamy Slag Fundamental and Their Practical Application to Electric Furnace Steelmaking. Iron and Steelmaker. 1999, 26, 79–88.
[8] Schürmann, E. and Kolm, I. Mathematische Beschreibung der MgO-Sättigung in Komplexen Stahlwerks-Schlacken beim Gleichgewicht mit Flüssigem Eisen. Steel Research. 1986, 57(1), 7–12.
[9] Park, J.M. and Lee K.K. Reaction Equilibria Between Liquid Iron and CaO-Al2O3-MgOsat.-SiO2-FetO-MnO-P2O5 Slag. 79th Steelmaking Conference, Pittsburgh, USA, March 24–27, 1996, 165–172.
[10] Park, J.M. MgO Solubility in BOF Slag Equilibrated with Ambient Air. Steel Research. 2001. 72, 141–145.
[11] Tayeb, M.A., Assis, A.N., Sridhar, S. and Fruehan, R.J. MgO Solubility in Steelmaking Slags. Metallurgical and Materials Transactions B. 2015. 46B, 1112–1114.
[12] Jung, S.M., Rhee, C.H. and Min, D.J. Solubility of MgO in CaO-Based Slags. Procemin 2008: V International Mineral Processing Seminar, Santiago, Chile, October 22–24, 2008, 133–142.
[13] Montecinos de Almeida, R.A., Vieira, D., Bielefeldt, W.V. and Faria Vilela, A.C. MgO Saturation Analysis of CaO-SiO2-FeO-MgO-Al2O3 Slag System. Materials Research. 2018, 21(1), e20170041.
[14] Khadhraoui, S., Hack, K., Jantzen, T. and Odenthal. H.-J. Study of the State of Industrial P2O5-Containing Slags Relevant to Steelmaking Processes Based on a New Thermodynamic Database Developed for CaO-FeOx-P2O5-SiO2-MnO-MgO-Al2O3 Slags, Part I: Ternary and Lower Order Systems. Steel Research International. 2019, June, 1900085.