- About
- Solutions
- Governance
- Investors
- Sustainability
- Sustainability
- Newsroom
- Jobs
To maintain and enhance the competitiveness of RHI Magnesita’s electric arc furnace (EAF) hearth ramming mixes (i.e., ANKERHARTH and PERRAMIT), continuous product optimisation is essential. This study addresses the necessary increase in calcium oxide (CaO) content resulting from the evolving composition of natural magnesite—particularly from the Hochfilzen mine in Austria. The objective is to ensure sustainable raw material utilisation while maintaining product quality and cost efficiency. Manufacturing flexibility across two Austrian sites (Breitenau and Hochfilzen) supports secure and adaptable supply. Extensive R&D—including laboratory testing, thermochemical modelling (FactSage), and phase diagram analysis—confirmed that the revised CaO content does not affect sintering behaviour, mechanical strength, or thermal performance. Field trials in AC and DC EAFs across multiple regions showed no differences in installation, wear, or repair practices. The adjusted formulation was fully implemented by the beginning of 2025. These findings validate a slight CaO adaptation as a sustainable, technically robust solution aligned with long-term refractory performance and customer reliability requirements.
In the last three decades, technological advancements in electric arc furnace (EAF) design and operation have transformed the EAF into a high-throughput, cost-efficient melting unit that plays a central role in the production of high-quality steel grades. These developments have been paralleled by a corresponding evolution in refractory technology, with modern EAF campaigns increasingly relying on tailor-engineered refractory solutions to withstand extreme thermal, chemical, and mechanical loads. Among the critical components of the EAF refractory system, the hearth ramming mix is especially significant, as it directly influences furnace safety, campaign longevity, and total cost of ownership.
The refractory design for ultra-high-power EAFs necessitates a holistic approach: It must account for regional operational conditions, employ materials with optimal thermomechanical and thermochemical performance, and minimise lifecycle refractory costs. For the hearth region specifically—where refractories are subjected to extreme thermal cycling, corrosive slag attack, melt penetration, and mechanical abrasion from scrap charging—these requirements are particularly stringent.
One of the most advanced ramming mixes developed to meet these operational demands is ANKERHARTH, a magnesia-based mix engineered for rapid sintering, high hot strength, and long service life. The performance of ANKERHARTH is underpinned by its precise raw material formulation and processing parameters. Key characteristics that distinguish it from conventional hearth mixes include:
The microstructural integrity of ANKERHARTH is critically influenced by its controlled phase composition—particularly the balance of periclase (MgO), lime (CaO), and iron oxide (Fe2O3)—and its tailored grain size distribution, which ensures high compaction during installation and minimised wear during operation. One of the principal mineralogical phases, dicalcium ferrite (Ca2Fe2O5), facilitates controlled low-temperature liquid phase formation (first eutectic melt at 1308 °C), which subsequently converts into high-melting-point compounds upon sintering, contributing to the robust ceramic matrix.
The success of ANKERHARTH is inherently tied to the use of Alpine sintered magnesia, sourced from the Hochfilzen and Breitenau mining and production operations in Austria. The geological profile of these mines, however, presents unique challenges. While Breitenau exhibits a wide variability in CaO concentration due to its combined open-pit and underground mining approach, Hochfilzen has, in recent years, demonstrated a marked shift toward higher CaO-content raw magnesite, particularly in residual reserves within rest pillars.
Without periodic reformulation of ANKERHARTH—specifically in 2005 and 2011—to adapt to the evolving mineralogy, the continued viability of mining operations at Hochfilzen would have been compromised. Maintaining the historical chemical specification of ANKERHARTH, while ignoring this shift, would have rendered long-term sustainable extraction unfeasible.
Therefore, to ensure responsible resource utilisation and uninterrupted supply to end-users, a strategic shift toward sustainable ANKERHARTH formulations is essential. This includes the carefully balanced increase of CaO levels in the mix design to align with the geological reality of the raw material base—without compromising the mix’s performance, sintering behaviour, or operational safety. This article describes the impact and optimisation potential of such a reformulation strategy, based on laboratory trials, plant-scale testing, and field validation at customer installations.
The CaO content in ANKERHARTH hearth mixes is a critical parameter that directly influences phase development during sintering and, consequently, the refractory’s performance in EAF service.
During raw material production in RHI Magnesita’s rotary kilns, CaO and Fe2O3 react to form Ca2Fe2O5—a key phase that drives early liquid-phase sintering. To promote Ca2Fe2O5 formation, a CaO/SiO2 ratio significantly greater than 2 is essential to ensure lime is available in sufficient quantity. Equally, or even more important, is the natural ore composition, particularly a homogeneous distribution of Fe2O3 within the Alpine magnesite feed. This ensures that Ca2Fe2O5 forms uniformly throughout the grain structure, preventing weak zones and inconsistent sintering (Figure 1).
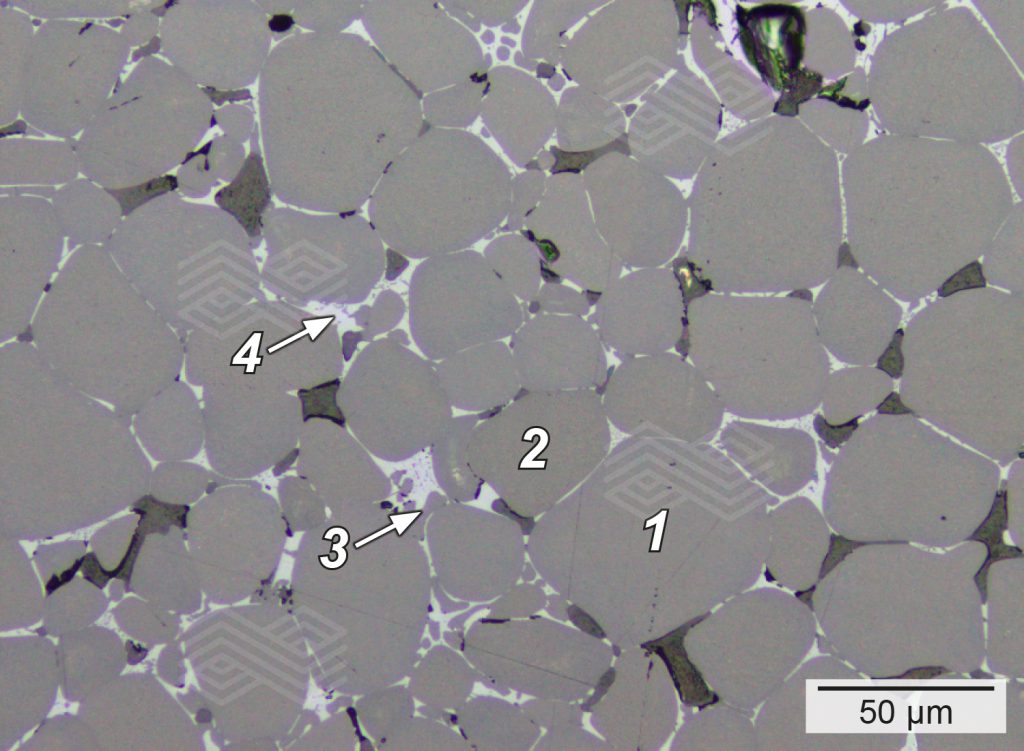
Figure 1. Micrograph illustrating a typical Alpine sintered magnesia grain used in ANKERHARTH, containing ~30% CaO and ~4.5% Fe2O3. The interstitial phases between periclase (1) and CaO (2) crystals are well-developed Ca2SiO4 (3) and Ca2Fe2O5 (4), confirming effective phase formation during sintering.
Synthetic alternatives, which combine low-iron MgO with limestone/dolomite, and added iron oxide, often fail to replicate the natural microstructural homogeneity of high-grade Alpine sintered magnesia. These engineered alternatives frequently exhibit inhomogeneous phase distribution, lower sinter density, and reduced resistance to slag attack and thermal cycling. As a result, their overall performance remains lower than that of natural Alpine magnesite-based materials.
In summary, the unique combination of high CaO content and naturally integrated Fe2O3 in Alpine magnesite is fundamental to the superior performance of ANKERHARTH hearth ramming mixes. This composition ensures robust sintering, mechanical integrity, and long service life in the harsh EAF hearth environment.
The sintering process during EAF start-up is crucial for developing the final microstructure and ensuring the mechanical integrity of the ANKERHARTH hearth ramming mix. This process, commonly referred to as the “ANKERHARTH effect”, is represented by the schematic equation 1 and is primarily driven by the transformation of Ca2Fe2O5 within the magnesia raw material. As a result, under reducing conditions, this reaction ultimately raises the invariant point of the mineral assemblage, from 1308–1386 °C (depending on the CaO/SiO2 ratio [2]) to 1850 °C, enabling enhanced high-temperature performance in the EAF.
Change from oxidising to reducing conditions at 1600 °C
MgO + Ca2Fe2O5 –> (Mg,Fe)O + CaO (1)
The formulated equation, or the so-called “ANKERHARTH effect”, can be explained in two steps:
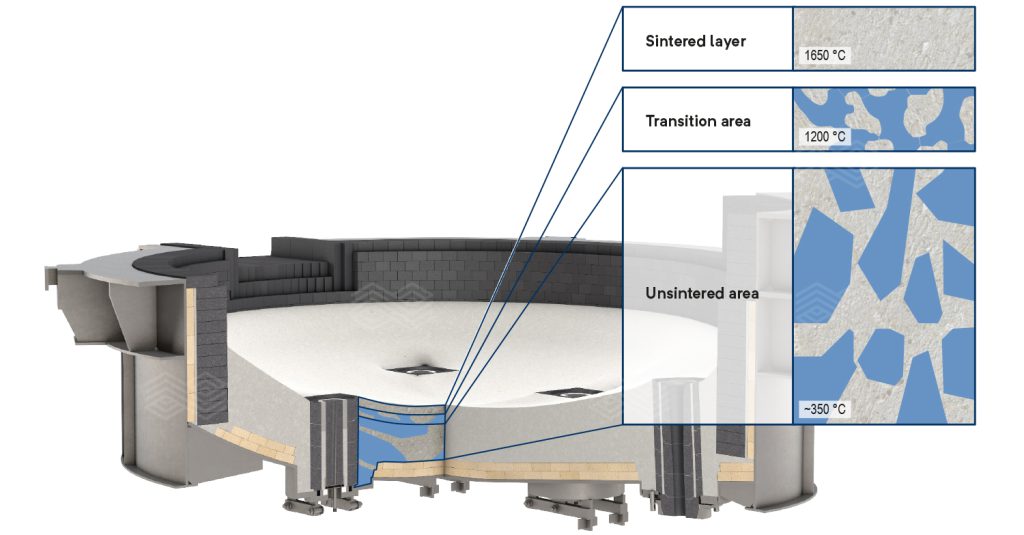
Figure 2. Illustration of the “ANKERHARTH effect”, showing the different temperature-dependent layers in the EAF hearth mix during heat-up and operation. Unsintered area: Periclase and Ca2Fe2O5 are both solid. Transition area: Solid periclase and newly formed interstitial liquid phase composed of CaO and Fe2O3, formed at the expense of primary Ca2Fe2O5. Sintered layer: Reduction of Fe3+ to Fe2+ under reducing conditions and formation of highly refractory solid magnesiowustite and CaO.
Although CaO levels in EAF hearth ramming mixes have traditionally been tightly controlled, a moderate increase in CaO content—aligned with raw material availability—does not negatively impact the performance of ANKERHARTH. On the contrary, it provides several thermochemical and structural benefits.
In terms of refractoriness and slag resistance, CaO exhibits a high melting point (~2600 °C) and excellent thermochemical stability, making it well suited for hightemperature applications. It also enhances resistance against acidic slags by modifying infiltration dynamics. When silica-rich slags penetrate and react with the refractory matrix, they typically form low-melting silicates. In high-Ca2Fe2O5 systems, however, increasing basicity with infiltration depth promotes Fe2O3 dissolution into periclase, which is a thermodynamically favourable process. Free CaO, not bound within Ca2Fe2O5, remains in the matrix and reacts with infiltrating SiO2 to form Ca2SiO4. The formation of Ca2SiO4 increases slag viscosity and contributes to pore blocking, thereby reducing further slag penetration. Field observations confirm that the stability of the binding phase—especially under slag infiltration—is a key determinant of refractory wear resistance.
Despite the elevated CaO, sufficient MgO remains present in ANKERHARTH mixes to maintain a robust crystalline scaffold. This MgO framework is essential for mechanical stability and cannot be achieved in pure dolomitic systems. Under reducing conditions, FeO from slag is absorbed into periclase, forming high-melting magnesiowustite. At the hot face, FeO and MnO further densify the surface zone by forming solid solution phases (Mg,Fe,Mn)O, improving resistance to chemical dissolution.
Consequently, high-Ca2Fe2O5 containing sintered magnesia mixes with slightly increased CaO not only preserve refractory integrity but also enhance performance under both acidic slag attack and reducing conditions—key challenges in modern EAF operations.
To validate the performance consistency of ANKERHARTH NN25 with elevated CaO content, comprehensive experimental and thermochemical investigations were conducted on both the standard and high-CaO ANKERHARTH NN25 mixes (Table I), combined with theoretical phase equilibrium analysis. This evaluation included a side-by-side comparison of mechanical properties, advanced phase equilibrium modelling using the Gibbs free energy minimisation software FactSage [3,4], and an evaluation of phase stability within the CaO–MgO–SiO2 (CMS) ternary system. For the samples used, elevated CaO levels compared to the values specified in the technical data sheet were applied to account for production variability and to simulate upper boundary conditions.
ANKERHARTH bottom ramming mixes used in EAFs are subjected to severe thermal, mechanical, and chemical loads. To evaluate their performance under such service conditions, three key parameters are routinely investigated at the Technology Center Leoben (Austria): Cold crushing strength (CCS), permanent linear change (PLC), and refractoriness under load (RUL). Together, these parameters characterise the material’s structural integrity and thermomechanical suitability for EAF applications. The CCS indicates the post-sintering mechanical stability of the material. High CCS values are typically associated with enhanced sintering and a dense microstructure, which increases resistance to slag attack and mechanical wear. However, in high-performance ANKERHARTH systems, excessively high CCS may be unfavourable. Intensified sintering can reduce the amount of unreacted (virgin) material in the ramming layer, which is necessary for long-term durability. Furthermore, deeper sintering correlates with increased thermal conductivity, potentially accelerating heat transfer to the furnace bottom plate and thereby reducing campaign lifetime due to elevated temperatures at the steel shell interface.
Conversely, insufficient CCS may indicate poor sintering or excessive porosity, compromising both mechanical strength and chemical resistance. Therefore, an optimal balance is required. CCS values above 50 N/mm2 at 1600 °C are generally considered suitable for high-performance EAF hearth applications, ensuring mechanical robustness without excessive heat conduction or loss of residual structure.
CCS measurements conducted at 800 °C, 1000 °C, 1300 °C, and 1600 °C demonstrated comparable performance between the standard and high-CaO formulations (Figure 3). Minor deviations in absolute values fell within typical sample variation and showed no trend of deterioration. Both formulations achieved optimal CCS at 1300 °C and 1600 °C indicating consistent mechanical integrity and sintering behaviour under high-temperature conditions representative of EAF operation.
PLC quantifies the dimensional stability of refractory materials under thermal load and is a key indicator of their high-temperature performance. Minimal irreversible length change during heating reflects superior thermal stability, minimising the risk of crack formation due to thermal stress. Refractory mixes exhibiting PLC values below 3.0% at 1600 °C are generally regarded as thermally stable, with consistent phase development and a homogeneous microstructure. This stability is essential for EAF hearth linings, which are frequently subjected to cyclic thermal loading due to operational interruptions and repair procedures.
PLC measurements confirmed the thermal dimensional stability of the high-CaO ANKERHARTH mix (Figure 4). Across all testing temperatures, both the standard and modified formulations exhibited comparable expansion and shrinkage behaviour, with no evidence of abnormal contraction or excessive swelling. These results verified that the CaO adaptation does not impair the product’s structural integrity during thermal cycling.
RUL evaluates the resistance of refractory materials to deformation under simultaneous exposure to high temperature and mechanical stress. This test replicates service conditions encountered in industrial furnace environments. The sample is subjected to a constant load of 0.2 MPa in a reducing atmosphere, while being heated at a controlled rate to a maximum temperature of 1600 °C.
The key metric is the T0.25 °C point, defined as the temperature at which the sample exhibits a 0.25% deformation relative to T0, the dimension at the onset of softening. This marks the formation of the first consistent sintering bridges and indicates the onset of structural consolidation under load.
RUL measurements for both the standard and high-CaO ANKERHARTH compositions showed converging softening behaviour, with equivalent T0.25 °C values and comparable deformation profiles under thermal load (Figure 5). These results confirmed that the increased CaO content does not reduce thermal resistance or compromise mechanical stability during high-temperature operation, particularly during initial sintering and early EAF heating cycles.
Thermochemical simulations were performed using FactSage 8.3 under both oxidising (pO2 = 0.21) and reducing (pO2 = 10-9) conditions. The modelled evolution of liquid phase formation up to 1600 °C showed near-complete overlap between the standard and high-CaO ANKERHARTH NN25 formulations under oxidising conditions (Figure 6). An analogous behaviour was evident in the simulation results under reducing conditions. This indicates that the initial sintering behaviour is unaffected by the increased CaO content during the first heating cycle.
Additionally, the impact of increased CaO on slag corrosion resistance was evaluated at 1700 °C using two representative slags: A typical EAF carbon steel slag and a typical EAF stainless steel slag, under both oxidising (pO2 = 0.21) and reducing (pO2 = 10-9) conditions. The simulations revealed no significant difference in total liquid phase formation between the standard and high-CaO ANKERHARTH NN25 mixes for oxidising conditions when in contact with an EAF carbon steel slag (Figure 7) and a typical EAF stainless steel slag. The same trend continued to be evident under reducing conditions when in contact with either of the two EAF slags. This thermodynamic equivalence confirms that the CaO-adjusted mix retains its chemical stability and integrity during both start-up and steady-state operation, even in contact with aggressive slag environments.
The derived invariant point in the CaO–MgO–SiO2 ternary phase diagram [5] confirmed that an increase in CaO content in the ANKERHARTH NN25 mix does not reduce the initial melting temperature. Both the standard formulation (CaO technical datasheet value of 23.0 wt.%) and the updated high-CaO formulation (CaO technical data sheet value of 26.5 wt.%) fall within the same conjugation triangle CaO–MgO–Ca3SiO5, corresponding to an invariant point at 1850 °C (Figure 8).
Even an increase to >30 wt.% CaO (indicated by the red dotted arrow in Figure 8), would remain within this triangle and retain the same initial melting point of 1850 °C. A lowering of the invariant point to 1790 °C would only occur if the SiO2 content were significantly increased, shifting the composition into the MgO–Ca3SiO5–Ca2SiO4 conjugation triangle.
Extensive tests were conducted across Europe and North America over several months to validate the compatibility and performance of the revised ANKERHARTH NN25 formulation and other ANKERHARTH grades. Multiple trial campaigns were executed, including “50/50 linings”, where half of the EAF hearth was lined with the standard mix and the other half with the updated high-CaO formulation. Both hot repair applications and complete relinings were carried out in AC and DC EAFs under varying process conditions. No adverse effects were reported regarding installation behaviour or in-service performance. Minor colour differences, observed by some customers, were attributed to the reduced iron content in the revised formulation but had no functional impact.
The transition to the updated formulation began in Q4 2024, following prior customer notification through direct communication and updated technical datasheets. All relevant internal and external stakeholders were aligned with the implementation plan to ensure a smooth rollout. Since Q1 2025, all regular customers have been supplied with material produced using the revised formulation.
At selected industrial locations, technical experts carried out comprehensive observations to assess operational performance. One campaign, conducted at a steel production facility with challenging process conditions, served as a reference case. Over a period of three months, different variations of the ANKERHARTH NN25 material were installed and monitored within a single operational cycle, reaching up to 1250 heats. The chemical composition of the various ANKERHARTH NN25 mixes (i.e., lower MgO, higher MgO, and customer-specific mid-range MgO) are detailed in Table II. The trial methodology was based on quantitative monitoring of material consumption, including initial lining and intermediate hot repairs.
The three different ANKERHARTH NN25 grades were closely monitored by an RHI Magnesita service technician stationed on-site, who was familiar with the specific process conditions and site circumstances. This ensured that all evaluations were consistent and meaningful. Compared to standard NN25 deliveries, the selection included one grade with lower MgO content (CaO content similar to the new technical data sheet specifications), one with higher MgO (i.e., low CaO content), and a mid-range variant, enabling potential performance differences to be identified.
Over a three-month period, each application followed a standardised procedure: Beginning with the cool-down phase, followed by new lining installation, intermediate taphole changes, and brick repairs, all combined with hot repair practices using the hearth mix itself. Performance was assessed by measuring residual height and calculating material consumption or loss throughout the operational cycles. These measurements covered various furnace zones, including the bottom, banks, taphole area, and slag door region. No irregularities were reported during lining or repair activities by either the technician or on-site personnel.
Campaigns of 1150 heats or more were run with each of the three mix alternatives, producing nearly 135000 tonnes of steel per campaign. These figures demonstrate that the trials were conducted under highly demanding conditions, given the large number of heats per campaign. For all three grades, the specific ANKERHARTH NN25 consumptions were between 0.85 and 0.87 kg per tonne of liquid steel. As shown in Table III, across all measurements, analyses, and observations, no significant deviations or fluctuations were observed between the three evaluated ANKERHARTH types. These results confirm that even at higher CaO concentrations, the performance of ANKERHARTH NN25 was equivalent to that of the former standard grade.
In response to the evolving chemical composition of domestic magnesite reserves—specifically the increasing CaO content in the Hochfilzen open-pit mine—this study evaluated the impact of elevated CaO levels in ANKERHARTH NN25 hearth ramming mixes used in EAFs. The objective was to enable sustainable raw material use while preserving refractory performance.
Through a combination of laboratory analysis (CCS, PLC, and RUL), thermochemical modelling (FactSage), and CaO-MgO-SiO2 phase diagram evaluation, the CaO-adapted formulations were shown to retain identical sintering behaviour, dimensional stability, and deformation resistance under load. Field validation across multiple industrial sites—including full-scale and 50/50 lining trials—confirmed compatibility under both AC and DC EAF operating conditions.
The transition to the revised formulation began in Q4 2024 and was completed by Q1 2025 without any reported negative performance. The adjusted chemical specification ensures long-term raw material sustainability and supply stability while maintaining refractory integrity, application reliability, and product quality. This optimisation supports cost-stable production and is reflected in the updated technical data sheet values of all ANKERHARTH products. The technical data sheet of ANKERHARTH NN25 is provided as an example in Figure 9.
[1] Eckstein, W., Zettl, K-M. and Wappel, D. ANKERHARTH—50th Anniversary of Electric Arc Furnace Bottom Ramming Mixes. RHI Bulletin. 2013, 8–13.
[2] Siegl, W. Dikalziumferritreiche Sintermagnesia in basischen Massen. Radex-Rundschau. 1989, 2/3, 99–117.
[3] Bale, C.W., Chartrand, P., Degterov, S.A., Eriksson, G., Hack, K., Ben Mahfoud, R., Melançon, J., Pelton, A.D. and Petersen, S. FactSage Thermochemical Software and Databases. Calphad. 2002, 26, 189–228.
[4] Bale, C.W., Bélisle, E., Chartrand, P., Decterov, S.A., Eriksson, G., Gheribi, A.E., Hack, K., Jung, I.-H., Kang, Y.-B., Melançon, J., Pelton, A.D., Petersen, S., Robelin C., Sangster, J., Spencer, P. and Van Ende, M-A. FactSage Thermochemical Software and Databases, 2010–2016. Calphad. 2016, 54, 35–53.
[5] Osborn, E.F. and Muan, A. Phase Equilibrium Diagrams of Oxide Systems, Plate 2; American Ceramic Society and the Edward Orton, Jr., Ceramic Foundation, 1960.